Johnson & Johnson reaches patients and consumers across the world with our medicines, medical technologies and consumer health products. Their safety when using our products is a critical priority for Johnson & Johnson: We insist on quality and safety at every stage of product development, manufacturing, supply chain and commercialization.
Carol Montandon
Global Vice President, Chief Quality Officer, Johnson & Johnson
Reinforcing our commitment to quality, all employees are required to take our interactive e-Learning Quality Management Framework course. The course informs employees about our quality management systems and provides examples of roles and responsibilities that help maintain quality across Johnson & Johnson and how everyone, in every role, should play a part.
Quality management
Our commitment to quality, safety and reliability is the basis for everything we do. Our Quality Policies and Quality Standards cover the lifecycle of our products from R&D to the patient and consumer experience. They provide a framework and common foundation of quality expectations and help ensure a reliable supply of high-quality products across all our business segments in the markets we serve.
In 2022, our Quality & Compliance organization led several improvements in our quality and compliance practices, including:
Transforming quality processes with digital solutions
Many tasks in Quality & Compliance are transactional, and to date, much employee time has been spent on manual processes. Automating manual tasks by applying intelligent analytical tools allows employees to tackle higher-value tasks such as data-driven proactive decision making and assessing larger data sets to further advance preventive quality management and create valuable product and patient insights. We continued to progress the digital transformation of our quality process and management systems in several ways, including:
- Installed a new data portal to increase access to Quality & Compliance data sources and improve agility through quality insights, analytical data tools and dashboards across all business segments.
- Achieved considerable efficiencies through the automation of manual processes, enabled by IA applications, in combination with data insights and natural language processing solutions.
- Developed new digital tools to assist in simplifying and improving the quality of literature, document and report reviews; capturing product insights from complaints; improving product release cycle time through transparency planning and managing sample and process flows.
Product Quality Indicators |
2022 |
2021 |
2020 |
||||||
Number of FDA warning letters issued |
0 |
0 |
0 |
||||||
Product recall rate* by business segment |
|||||||||
Pharmaceutical |
0.003%‡ |
0% |
0% |
||||||
MedTech |
0.005%‡ |
0.059% |
0.034% |
||||||
Consumer Health Self-Care OTC** |
0%‡ |
0% |
0% |
||||||
|
|||||||||
Refreshing our Quality & Compliance Competency Model
This model prioritizes upskilling our people to prepare them for proactive and capable quality management in a digitally enabled future. It encourages employees to actively participate in their individual career journey and promotes a culture of continuous and innovative learning.
Johnson & Johnson’s Quality & Compliance Competency Model has two core categories, nine core competencies and four proficiency levels per competency.
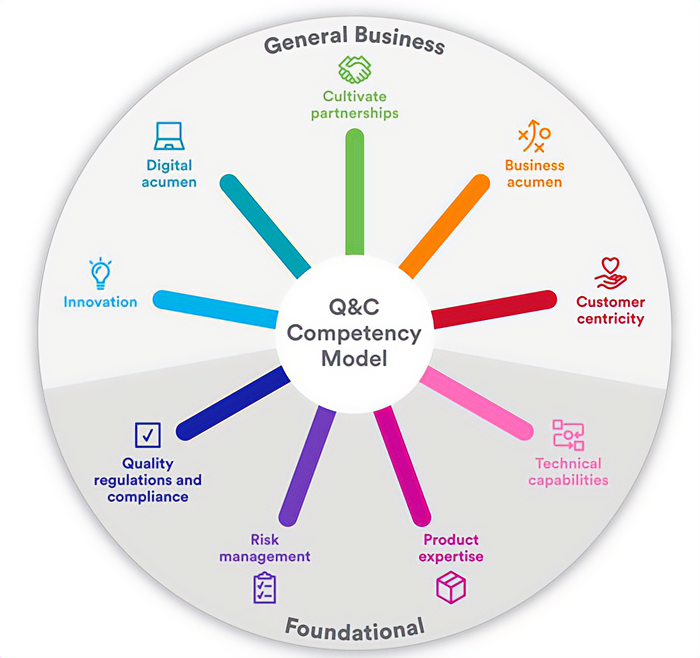
The Quality & Compliance Capability Framework, including the Competency Model, developed with wide collaboration across Johnson & Johnson, is aligned with our Enterprise approach to learning and development and provides an advanced platform to ensure our employees are developing within the critical capabilities needed most to deliver on our Quality & Compliance strategic goals, including strengthening the Quality & Compliance talent pipeline.
Introducing a new quality model for better customer experience
Customer Experience Engineering (CxE) is a new proactive quality model and capability that supports the involvement of our Quality & Compliance organization in technical improvements for products and services. CxE focuses entirely on the experiences of our customers and is led by internal Quality experts who can make a practical contribution to solving product challenges. With several projects in the pipeline, CxE promises to help transform the experience of patients, physicians and surgeons by enhancing our product and delivery quality with tangible customer outcomes. For example, in 2022, the Quality & Compliance CxE team engaged with the J&J MedTech DePuy Synthes team to solve a challenge relating to its PUREVUE Visualization System, which is used for arthroscopic procedures. When the system’s camera lens began to fog up during procedures, temporarily clouding the visualization of the operating area, the CxE team visited surgeons to witness the issue in real time in operating rooms and contributed to a new design solution that significantly improved the temporary visualization issue.
Reimagining Quality Month

Our theme for 2022 Quality Month was “Digitally Enabled End-to-End Quality,” which was supported by weekly topics that encouraged our global sites to focus on critical Quality & Compliance initiatives:
- Digital transformation: Using digital to enable our employees, customers and patients.
- Strategy: Leading the way in end-to-end patient, consumer and customer experiences enabled by a fully integrated digital ecosystem.
- Innovation: Innovating through the use of digital tools to enhance customer success.
- Learning: Driving learning and engagement beyond Quality Month to build a digitally enabled workforce for the future.
Employees around Johnson & Johnson engaged with our Quality Month in multiple in-person and virtual events across all our communication channels.








