At Johnson & Johnson, we have been committed to fighting HIV for decades. Together, we are helping to advance science, enable access and collaborate to improve lives around the world in a determined effort to make HIV history.
Though there have been significant advances in prevention since the beginning of the global epidemic, 1.5 million people acquired HIV in 2021 alone,28 underscoring the high unmet need for new options. That’s why we must continue to drive out inequalities in access to HIV treatment while making HIV treatment easier, more effective and more available for everyone.
Brian Woodfall, M.D.
Vice President, Global Head of Development, Infectious Diseases & Vaccines, Janssen R&D
New and expanded U.S. regulatory approvals for HIV treatment
Our fruitful collaboration with ViiV Healthcare has helped to deliver a new and meaningful treatment option, with the world’s first, complete long-acting injectable (LAI) HIV treatment regimen. The LAI regimen includes two ARV drugs, one developed by Janssen (rilpivirine) and the other by ViiV Healthcare (cabotegravir). This regimen is also known as CABENUVA (cabotegravir and rilpivirine) in the U.S. Both the once-monthly and the every-two-months versions of this HIV LAI treatment regimen for adults have been approved by health authorities in Europe, Switzerland and Canada. Additionally, the once-monthly version has been approved by the health authority in Australia.
In 2022, the FDA approved a label update for the product, giving HCPs and people living with HIV in the U.S. the option to start this once-monthly or every-two-month injectable treatment without the need for the oral lead-in phase. Additionally, in 2022, the FDA approved CABENUVA (cabotegravir and rilpivirine) for adolescents who are 12 years of age or older as the first and only LAI HIV regimen to be made available for eligible adolescents, reducing the daily pill burden through a treatment offered as few as six days per year. Adhering to treatment regimens can be difficult for adolescents, who may skip HIV medicine doses to hide their HIV-positive status from others.29
Kati Vandermeulen
Senior Director and Compound Development Team Leader, Infectious Diseases & Vaccines, Janssen R&D
Regulatory approvals and studies in resource-limited settings
In 2022, the HIV LAI regimen was approved by the Botswana Medicines Regulatory Authority, marking the first approval of our co-developed HIV LAI regimen in a resource-limited country. We continue to seek regulatory approvals in additional RLS, including regulatory submissions in South Africa in 2021 and Thailand in 2022.
Health for Humanity 2025 Goals
View ScorecardProgress in Access to HIV Treatment
- Registration approval was received from the Botswana Medicines Regulatory Authority, marking the first RLS approval of the co-developed HIV LAI regimen (Janssen’s rilpivirine LA with ViiV Healthcare’s cabotegravir LA).
- Clinical studies are ongoing to demonstrate the safety and efficacy of the HIV LAI regimen in adults and adolescents across multiple countries in sub-Saharan Africa.
On track
We are also conducting and/or supporting four key additional clinical studies to demonstrate the safety and efficacy (randomized controlled trials), as well as the feasibility and acceptability (implementation research), of the HIV LAI regimen in adults and adolescents across four sub-Saharan African countries: Kenya, South Africa, Uganda and Zimbabwe. All four studies are at different stages of enrolling more than a total of 1,700 participants:
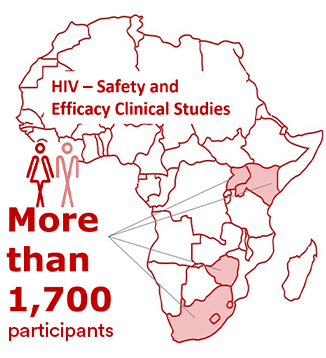
- Cabotegravir And Rilpivirine Efficacy and Safety Study (CARES study) in HIV-1 infected virologically suppressed adults;
- Long-Acting Treatment in Adolescents (LATA study) in HIV-1 infected virologically suppressed adolescents;
- Acceptability and Feasibility of Long-acting Injectable ART in Adolescents (AFINAty study) in HIV-1 infected virologically suppressed adolescents; and
- Improving HIV-1 Control in Africa With Long-Acting Antiretrovirals (IMPALA study) in HIV-1 infected adults with a history of suboptimal adherence.
Combating pediatric HIV
In addition to the FDA approval of the HIV LAI regimen for adolescents, our work to expand improved solutions for children living with HIV is ongoing. A combination tablet, containing our product PREZISTA (darunavir) in a fixed-dose combination with ritonavir 50mg, received WHO Prequalification in 2022 and is helping meet treatment needs as a second-line treatment for adults and children. Johnson & Johnson has had a specific policy since 2012 not to enforce the patents it owns and controls on PREZISTA (darunavir) in sub-Saharan Africa and Least Developed Countries. In 2015, we expanded the geographical scope of the policy for pediatric products used in LMICs.
In 2021, children accounted for 4% of all people living with HIV but for 15% of all AIDS-related deaths.30

Johnson & Johnson is a co-founder of the New Horizons Collaborative, which aims to improve treatment and care for children and young adults with HIV in 11 countries across the region through drug donation and capacity building. In 2021, Johnson & Johnson, in collaboration with the Elizabeth Glaser Pediatric AIDS Foundation,31 renewed our commitment to the initiative, pledging to extend our darunavir donation program and continue enrolling new pediatric and young adult HIV patients through at least 2025. This is also an important step toward meeting our commitments to help tackle the challenge of pediatric HIV as part of the Rome 5 Paediatric HIV and TB Action Plan. In 2022, more than 1,700 children were enrolled in this treatment program in sub-Saharan African countries.
HIV prevention for women and girls
The dapivirine vaginal ring, recommended in 2021 by the WHO as an additional prevention choice for women as part of a combination of prevention approaches, received approval in 2022 from the South African Health Products Regulatory Authority for use by women aged 18 and older to help reduce their risk of contracting HIV. Several approvals in Africa have been received, and there are additional applications currently pending.

The effects of gender inequalities on women’s HIV risks are especially pronounced in sub-Saharan Africa, where women accounted for 63% of new HIV infections in 2021.32
A mother and daughter wait to be tested at an HIV clinic in Kihihi, Uganda. Photo by New Horizons Collaborative.
32 UNAIDS, “Dangerous Inequalities, World AIDS Day Report 2022,” https://www.unaids.org/sites/default/files/media_asset/dangerous-inequalities_en.pdf, accessed February 2023.
The dapivirine ring is a long-acting HIV prevention method designed specifically for women. It was developed by the International Partnership for Microbicides under an exclusive license from Janssen for use of its ARV compound and is now owned by Population Council. The flexible, silicone ring is discreet, easy to use, has minimal side effects and only needs to be replaced monthly. The initial approvals and pilot implementation of the dapivirine ring in South Africa is an important step in the decades-long fight against HIV, particularly for adolescent girls and young women in sub-Saharan Africa, who are three times more likely to acquire HIV than their male counterparts.33
Johnson & Johnson was a founding member of the MenStar Coalition, which has helped more than 3 million men living with HIV in sub-Saharan Africa get into treatment, with 95% becoming virally suppressed.
Learning from HIV vaccine trials
In January 2023, together with a consortium of global partners, we discontinued Mosaico, our large-scale efficacy study in the Americas and Europe, that aimed to test the safety and efficacy of a specific composition of an investigational HIV vaccine regimen among men who have sex with men and transgender individuals. The study’s independent Data and Safety Monitoring Board determined that the regimen was not effective in preventing HIV infection compared to placebo among study participants. No vaccine-related safety issues were identified.
Penny Heaton, M.D.
Global Therapeutic Area Head, Infectious Diseases & Vaccines, Janssen R&D
28 UNAIDS, “Global HIV & AIDS statistics — Fact sheet 2022,” accessed February 2023.
29 HIV.info, NIH.gov, “HIV and Children and Adolescents,” accessed February 2023.
30 UNAIDS, “Dangerous Inequalities, World AIDS Day Report 2022,” https://www.unaids.org/sites/default/files/media_asset/dangerous-inequalities_en.pdf, accessed February 2023.
31 Funded primarily by the Johnson & Johnson Foundation, a registered charity and a company limited by guarantee. The Foundation is a separate legal entity from the Johnson & Johnson Family of Companies. The Foundation operates worldwide as Johnson & Johnson Foundation United States (founded 1953) and Johnson & Johnson Foundation Scotland (founded 2007).
33 Ibid.








