For more than a century, we have harnessed the power of technology and breakthrough innovation to elevate new standards of care. In surgery, orthopaedics, heart rhythm disorders, eye health, neurovascular care and other fields, we help save and improve lives and create a future where healthcare solutions are smarter, less invasive, and more personalized.
In a period of expansion and innovation, J&J MedTech delivered more than 30 significant product launches in the past two years, bringing advanced medical technologies to more people around the world.
Ahmet Tezel
Company Group Chairman and Global Head of MedTech Innovation and R&D, Johnson & Johnson
New patient-centered innovation
Across our wide range of innovative surgical technologies and solutions for patients and HCPs, we seek continuous improvement to deliver better patient outcomes with safety, efficiency and reliability. Examples from 2022 include:
- Part of J&J MedTech’s DePuy Synthes’ VELYS Digital Surgery Platform, CUPTIMIZE Hip-Spine Analysis is the latest surgical planning feature in VELYS Hip Navigation. It is a simple, x-ray-based digital tool that uses patient specific data to help surgeons assess dislocation risk and identify patients with abnormal pelvic tilt who may require unique cup placement or dual mobility design.
- DePuy Synthes specializes in orthopaedics solutions to help heal and restore movement for millions of patients. In China, working with Johnson & Johnson’s 3D Printing Group, DePuy Synthes is bringing to the Chinese market a highly personalized 3D-printed cranial implant made from a thermoplastic polymer. 3D execution enables the availability of these critical implants at a much lower cost and with shorter lead times than implants manufactured traditionally to help ensure that every surgery is safe, precise and time efficient. Production of the cranial implants is now up and running at the DePuy Synthes Suzhou Manufacturing Plant.
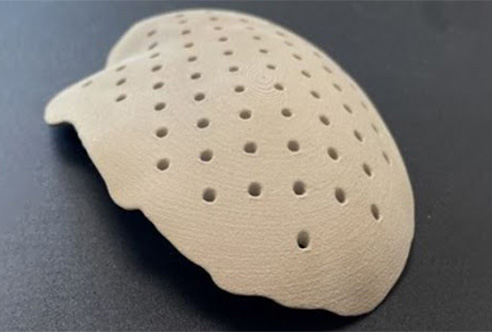
A 3D-printed cranial plate sample produced by Johnson & Johnson’s 3D Printing Group.
- J&J MedTech’s Ethicon franchise received FDA clearance for urology procedures using the MONARCH Endoscopic Robotic Platform. MONARCH is now the first and only multispecialty, flexible robotic solution for use in both bronchoscopy and urology.
- Ethicon continued to advance a next-generation treatment option for lung tumors to minimize the impact of surgery for patients and allow for speedier recovery. Ethicon’s solution is through transbronchial microwave ablation using its NEUWAVE FLEX Microwave Ablation System guided by its MONARCH Platform. Ethicon’s clinical trial for patients with lung tumors, the POWER study, aims to demonstrate the safety and effectiveness of this innovative approach. In December 2022, a milestone was achieved with the first patient successfully treated as part of the POWER study.
Toward the close of December 2022, we completed our acquisition of Abiomed, Inc., a world leader in heart recovery solutions and the creator of the world’s first totally implantable artificial heart. Abiomed brings a rich history of innovation in cardiovascular care that complements our existing leadership in the diagnosis and treatment of cardiac arrhythmias through our Biosense Webster electrophysiology business.
Advancing treatment of stroke
We collaborate with physicians and academics and invest in scientific research to advance the treatment of ischemic and hemorrhagic stroke. Stroke is a leading cause of death with 15 million people worldwide affected by stroke annually.47 In 2022, Cerenovus, a J&J MedTech company, launched the EMBOGUARD Balloon Guide Catheter as part of the CERENOVUS Stroke Solutions suite of technologies. EMBOGUARD is designed to optimize the removal of blood clots by controlling blood flow locally during thrombectomy procedures. Balloon guide catheters have been shown to increase the ability to restore blood flow after one pass, reduce procedure time, and reduce the odds of clot fragments breaking off and causing new ischemic events.48
Preventing and treating atrial fibrillation (AFib)
We advanced solutions to address AFib with new products, research and awareness efforts to inform and help patients, including:
- In Europe, Biosense Webster launched its first radiofrequency balloon ablation catheter, HELIOSTAR, to enable physicians in Europe to advance safe, effective and efficient treatment solutions for patients suffering from AFib. In Europe, catheter ablation is a recommended first-line treatment option.49
- OCTARAY Mapping Catheter was launched for mapping cardiac arrhythmias, including AFib, to assist physicians in capturing precise information for catheter ablation procedures with enhanced clarity and speed.
Dr. Amit Thosani
Director of Cardiac Electrophysiology, Allegheny Health Network
AFib is the most frequent cardiac arrhythmia. It has been estimated that 6 to 12 million people worldwide will suffer with this condition in the U.S. by 2050 and 17.9 million people in Europe by 2060.50
- In April 2022, Biosense Webster announced that the first patients were enrolled in the admIRE clinical trial in the U.S. to evaluate the safety and effectiveness of the company’s investigational Pulsed Field Ablation (PFA) platform, the principal components of which are the VARIPULSE catheter and the TRUPULSE generator, for the treatment of AFib. PFA represents a new approach to treating AFib, utilizing a controlled electric field—instead of thermal energy—to ablate and treat cardiac tissue, which could be an exciting advance for physicians and patients and may have a variety of benefits. Biosense Webster is working to bring a versatile portfolio of PFA solutions—complementary to our RF catheter portfolio—to address various ablation strategies in the treatment of AFib.
- J&J MedTech company Biosense Webster, progressed research into AFib patient behaviors in North America to help inform ways to support AFib prevention and treatment by physicians and patients. Biosense Webster’s AFib PulseCheck Survey among adults diagnosed with AFib revealed that, despite fears and anxiety about AFib, patients delayed seeking treatment, and many ended up in the emergency room due to AFib. These insights provide clues as to how best to promote AFib behavior change, especially among high-risk patients.
- In China, Biosense Webster supported a new Starlight AFib Public Awareness Campaign to help popularize knowledge on the prevention and treatment of AFib. As the top cause of death for the elderly, cardiovascular diseases are becoming a major health threat to Chinese people.
- Biosense Webster built the largest online patient community with the global Get Smart About AFib campaign. In the U.S. alone, there are more than 100,000 participants. This effort is dedicated to educating patients, caregivers and healthcare providers about AFib signs, symptoms and treatment options, including catheter ablation.
Sun Zhiwei
Chairman, Beijing Bethune Charitable Foundation
47 WHO, “Stroke, Cerebrovascular accident,” https://www.emro.who.int/health-topics/stroke-cerebrovascular-accident/index.html, accessed April 2023.
48 J&J MedTech, “CERENOVUS Launches EMBOGUARD™ Balloon Guide Catheter to Use In the Treatment of Acute Ischemic Stroke,” https://www.jnjmedtech.com/en-US/news-events/cerenovus-launches-emboguard-balloon-guide-catheter-use-treatment-acute-ischemic.
49 Hindricks G, Potpara T, Dagres N, et al, “2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS),” Euro Heart J, 2021 Feb 1;42(5):373-498, https://pubmed.ncbi.nlm.nih.gov/32860505, accessed February 2023.
50 Lippi G, Sanchis-Gomar S, and Cervallin G, “Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge,” Int J Stroke, 2021 Feb;16(2):217-221, https://pubmed.ncbi.nlm.nih.gov/31955707, accessed February 2023.








