Neglected tropical diseases (NTDs) are a cause of significant illness, disability and death, especially among children in some of the world’s poorest and most vulnerable communities.
In 2022, we renewed our commitment to fight NTDs by joining the global community, including governments, foundations, nonprofit organizations and other pharmaceutical companies, to endorse the Kigali Declaration on Neglected Tropical Diseases. Together, we are recommitting to our long-established efforts to control and eliminate NTDs, a set of debilitating diseases that put at risk the health of 1.7 billion people43 around the world, especially the most vulnerable and under-resourced. We committed to the following:
- donating up to 200 million doses annually of VERMOX (mebendazole), our medicine to treat intestinal worms, through 2025; and
- advancing novel R&D programs to discover new medicines needed to beat dengue and leprosy.
Of the 20 NTDs prioritized in the WHO roadmap 2021–2030, Johnson & Johnson research and medicines support the treatment and prevention of three high-prevalence NTDs: dengue; soil-transmitted helminthiasis (STH), also known as intestinal worms; and leprosy.44
Donating more than 2.2 billion doses of VERMOX (mebendazole)
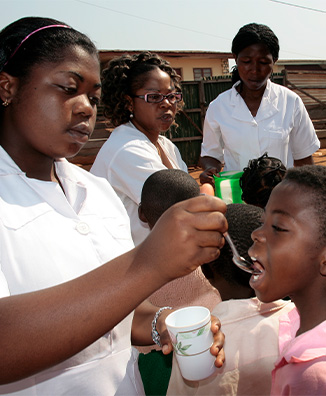
For more than 15 years, Johnson & Johnson has been at the forefront of global efforts to beat NTDs, donating more than 2.2 billion doses of VERMOX (mebendazole) for the treatment of intestinal worms to field partners in more than 60 countries. This was further bolstered by the development of a new chewable formulation of VERMOX (mebendazole) in 2019 that can be easily administered to children as young as 1 year of age, supporting the training of community health workers, enabling better data management and supporting the development of new diagnostics. Through tireless and collaborative efforts, countless children in need have been given the opportunity to recover, play, learn and thrive.
Since 2006, the VERMOX (mebendazole) program donated more than 2.2 billion doses, treating up to 100 million patients annually in 61 countries. More than 200 million doses of VERMOX Chewable were donated in 2022 alone.
Innovation to combat flavivirus
We also invited scientists from across the APAC and Oceania regions to take part in our Global Health Discovery QuickFire Challenge: Flavivirus Infections. The challenge aimed to attract potentially groundbreaking ideas to treat, control or prevent flavivirus disease. The innovator(s) with the best potential solution receive grant funding from a total pool of up to $300,000; access to the JLABS APAC community, including a dedicated workstation for one year at JLABS @ Shanghai; and mentorship from Johnson & Johnson experts.
Treating leprosy
Preliminary data from Janssen’s Phase 2a proof-of-concept study show promising results for bedaquiline for the treatment of leprosy. We shared these results at the 21st International Leprosy Congress 2022, noting that our compound bedaquiline appears to be generally well tolerated when dosed as monotherapy over a period of eight weeks in a leprosy study population consisting of multibacillary leprosy patients, the most advanced form of leprosy, followed by 12 months of a WHO-recommended leprosy treatment regimen. The preliminary data mark an important milestone in Johnson & Johnson’s pledge to advance research and development programs and generate the evidence needed to explore innovative treatment options to tackle leprosy.
Progressing dengue treatment and research
Janssen’s Dengue Compound Discovery Program, which started in 2007 in collaboration with KU Leuven Rega Institute and the Centre for Drug Design and Discovery in Belgium, has developed a dengue-specific antiviral compound with a novel mechanism of action that could potentially protect against and treat all dengue serotypes. Dengue eradication or elimination is not feasible due to existing animal reservoirs and limitations of current protective measures against the multiple strains and serotypes circulating. The toolbox to combat dengue needs to grow, and antivirals could be a vital new tool, helping to slow down outbreaks and limit epidemic levels of cases.
Upon proof-of-concept in a non-human primate model, and following results from our first-in-human Phase 1 double-blind study that showed the compound is safe and well tolerated, we progressed in 2022 to Phase 2a clinical studies to evaluate our antiviral compound for prevention and treatment of dengue. One study is a human challenge study conducted in collaboration with Johns Hopkins University, University of Vermont and NIH. The other is being conducted in collaboration with Singapore’s Duke-NUS Medical School, which is also home to Johnson & Johnson’s third Satellite Center for Global Health Discovery in APAC, launched in June 2022. This Satellite Center aims to catalyze investment and bolster R&D infrastructure for flaviviruses and builds on Johnson & Johnson’s decade-long legacy and ongoing collaboration with Duke-NUS in public health research.
Professor Patrick Casey
Senior Vice Dean of Research, Duke-NUS Medical School
43https://unitingtocombatntds.org/en/the-kigali-declaration/the-declaration/, accessed February 2023.
44WHO, Ending the neglect to attain Sustainable Development Goals: A road map for neglected tropical diseases, 2021–2030,” https://apps.who.int/iris/rest/bitstreams/1326801/retrieve, accessed February 2023.








